SYNDROME
|
Home
International Crigler-Najjar Registry and Associations
|
BilirubinBilirubin is the main waste product that results from hemoglobin breakdown during normal red blood cells turnover. There are two forms of circulating bilirubin: unconjugated (indirect) and conjugated (direct) bilirubin.
Total serum bilirubin (referred as total bilirubin) equals the sum of direct (conjugated) and indirect (unconjugated) bilirubin.
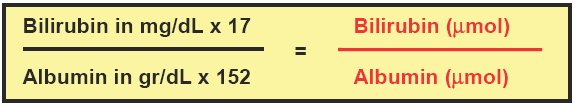
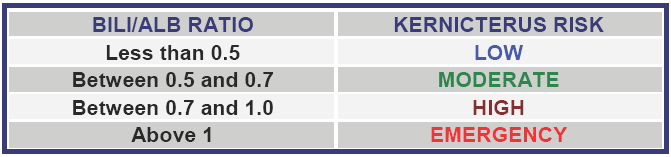
Bilirubin ToxicityAcute Toxicity:Current therapy (phototherapy, phenobarbital, cholestyramine, calcium phosphate) is useful to maintain fairly stable hematic levels of bilirubin in Crigler-Najjar patients. Nevertheless, a dramatic increase of bilirubin levels due to different causes is always possible. It is believed when levels of bilirubin are above 500 micromoles/L (i.e. about 30 mg/dL) the risk of acute toxicity is maximal, especially if the rise of the values is rapid.Moreover, levels between 300-500 micromoles/L (i.e. 17.5-30 mg/dL) are already at risk of chronic toxicity. Therefore, the optimal level should be around 200-300 micromoles/L (12-18 mg/dL) and in Crigler-Najjar type I patients values have to be maintained within this range or below.Dr. Morton (Clinic for Special Children, Strasburg, Pennsylvania) has pointed out that the risk of kernicterus is generally associated with an increase of the molar ratio between bilirubin and albumin (bili/alb) to >1, while its normal reference value is <0.6.How to calculate the bilirubin-albumin molar ratio.
Correlation between the value of the bilirubin-albumin molar ratio and risk of kernicterus (from Strauss et al.). The increase of the bili/alb ratio could be the result both of an increase of bilirubin and a decrease of albumin.Possible causes of an increase of bilirubin are:
Possible causes of a decrease of albumin are:
Some drugs could also increase the risk of acute bilirubin toxicity: some drugs may compete with albumin binding sites (ex: ceftriaxone, that is Rocephin); other drugs can cause direct hepatotoxicity (ex: valproate, that is Depacon); others can damage mitochondria, such as aspirin. For more details click here.To face a rapid increase of bilirubin is necessary to perform bilirubin and albumin analyses and calculate the bili/alb ratio.If the ratio bili/alb increases from 0.6 to 1:1. If the patient is already in phototherapy treatment increase the duration to 24 hours/day; if the patient is affected by Crigler-Najjar syndrome type II and does not undergo phototherapy but just phenobarbital treatment, start immediately a phototherapy treatment.2. i.v. injection of albumin (1-2 gr/kg).3. If neurologic symptoms have already been developed (torpor, sleepiness, alterations of the sleep waking rhythm, disruption of the equilibrium with dismetria and ataxia, slurring of speech, altered writing and convulsive attacks) perform plasmapheresis.It could be useful to use tin-mesoporhpyrin, an inhibitor of the enzyme heme-oxygenase.Chronic Toxicity:Symptoms are generally manifested when the bilirubin values are maintained between 17.5 and 30 mg/dL, but can also show up at lower values (es: 15 mg/dL) and with a bili/alb ratio < 0.5.They are:
There is no actual therapy for such symptoms, however, metabolic co-factors protecting mitochondria and supplement in energetic contribution could be helpful. Of course, the efficiency of the therapies in action (efficiency of the lamps, dosage of the Phenobarbital etc.) must be verified.Moreover, the appearance of symptoms of chronic toxicity in a previously asymptomatic patient can indicate a rising trend of the bilirubin values and, therefore, demand more frequent controls of bilirubin levels.A small increase of transaminases (AST, ALT) also indicates the toxic effect of the bilirubin on the liver. High concentration of bilirubin in the bile facilitates the formation of stones in the cholecystis. At the same time they can hinder the bile flux and favor the onset of acute cholecystitis. Such pathology can threaten the life of the Crigler-Najjar patient.Among patients in phototherapy from USA and Netherlands the rate of formation of stones in the cholecystis is above 50%. Most of these patients have been treated with laparoscopic cholecystectomy. Such an high frequency of formation of stones has never been observed in patients from France and Italy.To prevent the formation of stones it could be helpful to use ursodeoxycholic acid (Ursodiol), 20-30 mg/day. Before starting this therapy it is better to check the presence of stones in the cholecystis by echography.The Chronic Therapy :Crigler-Najjar syndrome type I:The main therapy is phototherapy using lamps that emit light with appropriate wavelengths (420-460 nanometers). It is carried out during the night (and when needed also during the day). The lamps generally used are Philips F20, F40 Special Blue lamps or Philips TL 52 lamps; their duration is around 1500 hours, after that their efficiency is reduced. There are no substantial side effects to their use, even if it is possible to onset photo dermatitis. Unfortunately, the effectiveness of phototherapy is reduced with the age because of the thickening of the skin, its pigmentation, and possible post-puberal hormonal variations. Moreover it is generally difficult to impose long phototherapy sessions to teen-agers due to the limits connected with such therapy.The light acts to transform the bilirubin in its photoisomers which are water soluble and, therefore, excreted through alternative ways. Its effectiveness can be increased by some drugs which link bilirubin in the intestinal lumen, avoiding the re-uptake and the return in the blood circle, increasing the elimination with feces.Such drugs are:- Cholestyramine: (Questran): 3-18 gr/die. Its use demands periodic (2/year) coagulation tests and possible vitamin K supplement.- Calcium-Phospate: there are not specific evidences of its chronic use. The preparation is a mixture of calcium - phosphate and calcium carbonate (2.5 mmol/kg, max. 100 mmol/die). Periodic controls of calcemia, phosphoremia, alkaline phosphatase are opportune. Potential risk of calculosis of the urinary tract.Phenobarbital is not active in the Crigler-Najjar type I patients (the enzyme is completely absent).Crigler-Najjar syndrome type II:The enzymatic deficit is partial and an induction of the enzyme activity can be obtained with the use of Phenobarbital (3-5 mg/kg/die). The best effect is obtained with levels of barbituremia (barbiturate levels) of at least 20 mg/dL.The response to phenobarbital treatment differentiates Crigler-Najjar syndrome type I, in which there is no response, from Crigler-Najjar syndrome type II. In type II at the beginning of such therapy cause a reduction of the bilirubin level of at least 20%, regardless the levels at the starting point. However, the chronic use of the drug has disadvantages, and some patients have to be treated with phototherapy. Moreover phototherapy may be necessary, since some Crigler-Najjar type II have levels of bilirubin analogous to Crigler-Najjar type I and can, therefore, have hyperbilirubinemic peaks.Periodic controls:It is opportune to repeat 2 or 3 times per year some controls:
Moreover, it is better perform annually an hepatic echography and an echography of the biliary ducts as well as an EEG in order to estimate the possible appearance of anomalous graphoelements that could be signs of damage due to bilirubin.Conversions of the values:
Note: the source of conversion factors is The European Journal of Pediatrics, Strauss et al. 2006
|
 Double click on any word of the text of this page to have its definition.
Double click on any word of the text of this page to have its definition.